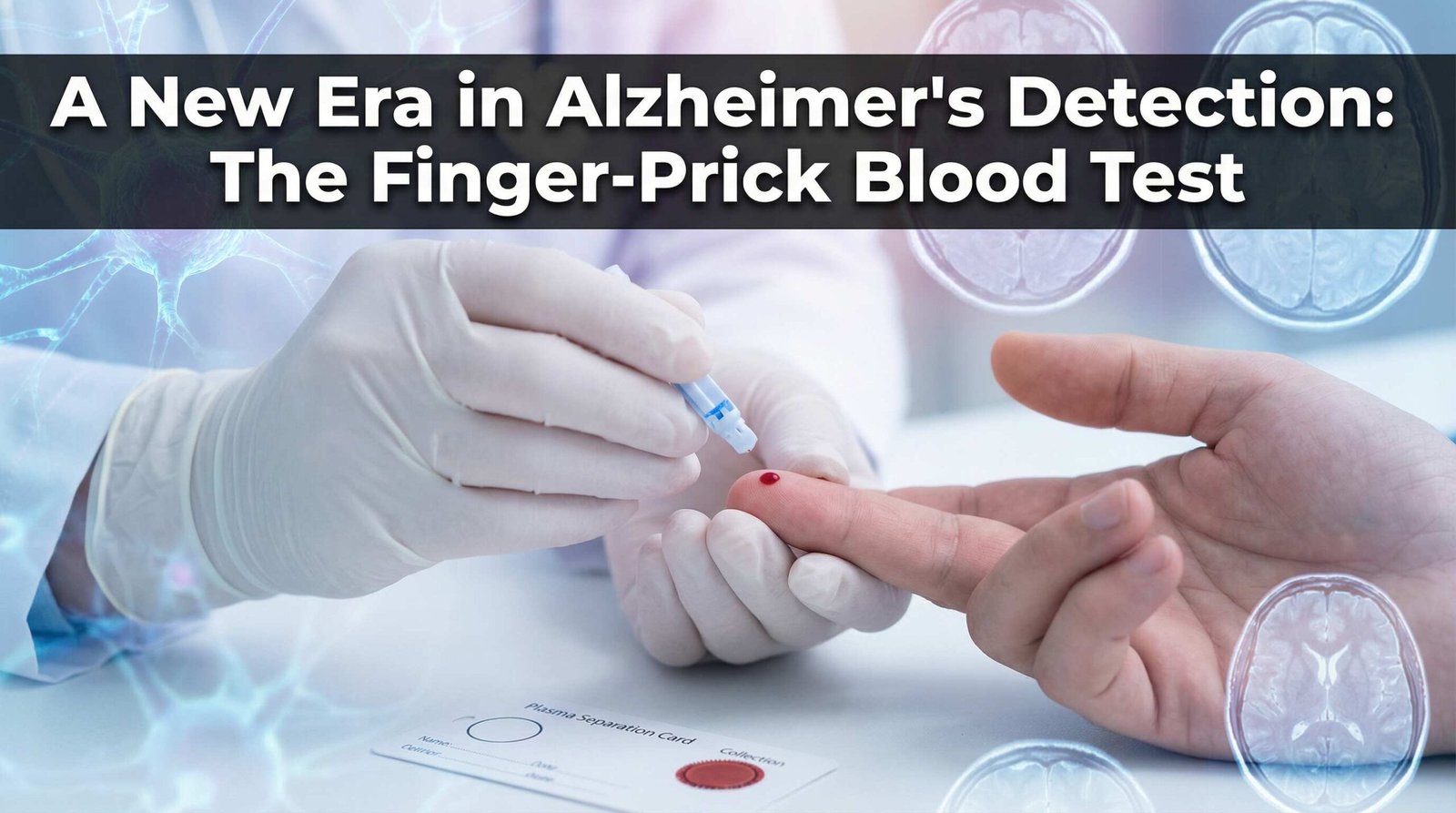
Could a simple finger-prick blood test change how we diagnose Alzheimer’s disease? A major international research project is underway to find out. This new test could make early detection more accessible and affordable for everyone.
The Challenge of Early Diagnosis
Currently, diagnosing Alzheimer’s disease is a complex process. It often involves expensive brain scans and invasive procedures like spinal taps. These methods are not only costly but also inaccessible to many people, especially those in areas with limited healthcare resources.
This creates a significant barrier to early diagnosis. Early detection is crucial because new treatments are most effective in the early stages of the disease. A delay in diagnosis can mean a missed opportunity to slow the progression of Alzheimer’s.
A New Hope: The Finger-Prick Test
A new finger-prick blood test offers a promising solution. This test is simple, and can be done almost anywhere. It uses a special card to separate plasma from the blood, which can then be sent to a lab for analysis without needing refrigeration.
This makes the test much cheaper and easier to use than current methods. If successful, it could provide a scalable and cost-effective way to screen for Alzheimer’s disease. This would allow for earlier intervention and a better chance of delaying the onset of symptoms.
The Bio-Hermes-002 Study
The finger-prick test is being evaluated in the Bio-Hermes-002 study, led by the Global Alzheimer’s Platform Foundation (GAP). This study involves 1,000 participants from the UK, USA, and Canada. The participants include people who are cognitively normal, those with mild cognitive impairment, and individuals with mild to moderate Alzheimer’s disease.
| Study Snapshot | Details |
| Study Name | Bio-Hermes-002 |
| Led by | Global Alzheimer’s Platform Foundation (GAP) |
| Partners | LifeArc, UK Dementia Research Institute (UK DRI) |
| Participants | 1,000 (883 enrolled) |
| Locations | 25 sites in UK, USA, Canada |
| Expected Completion | 2028 |
The Science Behind the Test
The test looks for three specific proteins in the blood that are associated with Alzheimer’s disease. These proteins are known as biomarkers.
•Phosphorylated tau 217 (pTau217): A key indicator of the tau tangles that are a hallmark of Alzheimer’s.
•Glial fibrillary acidic protein (GFAP): A protein that is released when brain cells are damaged.
•Neurofilament light polypeptide (NfL): Another protein that indicates nerve cell damage.
The results of the finger-prick test will be compared with the current gold standard diagnostic methods, including PET scans and MRI scans. This will help to validate the accuracy of the new test.
A More Inclusive Future
The Bio-Hermes-002 study is also committed to diversity. At least 25% of the participants will be from under-represented ethnic groups. This is important because Black and Hispanic people are twice as likely to develop Alzheimer’s disease as white people, yet they are often underrepresented in clinical trials.
By including a diverse group of participants, the study will help to ensure that future diagnostic tools and treatments are effective for everyone.
Looking Ahead
While more research is needed before this test can be widely used, the Bio-Hermes-002 study is a significant step forward. A simple, affordable, and accessible blood test for Alzheimer’s disease could revolutionize how we diagnose and manage this condition.
It could empower more people to get an early diagnosis and access the latest treatments. This could give them more time to live their lives to the fullest.